Cleaning an incubator shaker sounds trivial but is critical for successful cultivation. The good news is that doing the right thing is simple, effective and can easily be integrated into the routine management of a biosciences laboratory.

The key points covered in the following article are:
- The different levels of cleaning and disinfection
- What cleaning agents to use and their proper application
- Specific cleaning advice on individual components of the incubator shaker
- Hard surfaces inside and outside the shaker
- Under the tray
- Adhesive matting
- Fans and electronics
- Around the shaker
- Hard surfaces inside and outside the shaker
Different levels of cleaning and decontamination
Sanitisation is the most common solution for a routine clean. Disinfection should deal with most simple cases of contamination and sterilisation is reserved for serious problems not solved by the two lesser categories, or to address the requirement for certification of decontamination.
- Sanitisation – surfaces cleaned and microbial load reduced
- Disinfection – microbes on surfaced killed, inactivated and made harmless
- Sterilisation – total removal of any contamination so no viable microbes (or spores) remain
What cleaning agents to use
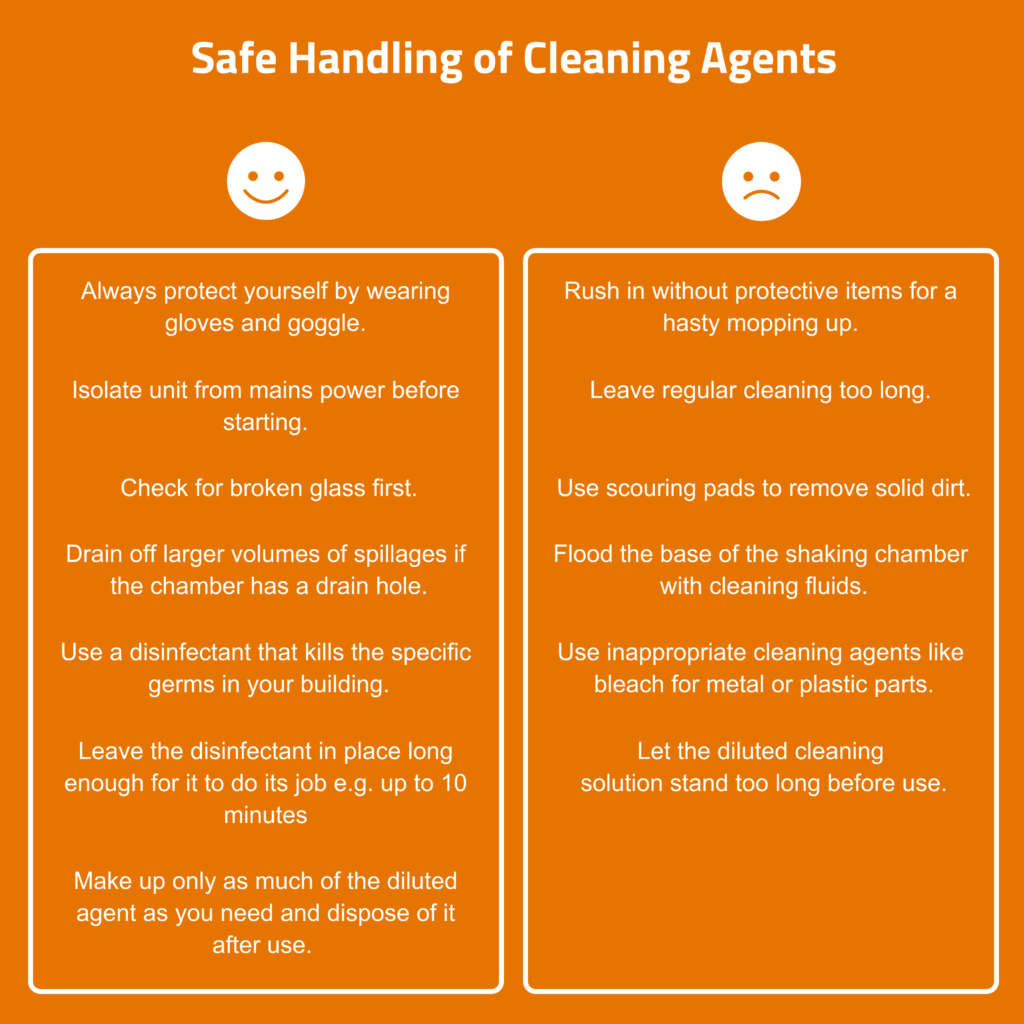
A ‘mild cleaning agent’ such as soap or household detergent will clean greasy surfaces but may have little antimicrobial activity. It can be applied with a soft, lint-free cloth such as microfibre, although unwanted residue may be created and an additional rinse may be required. Many generic ‘quaternary ammonium compounds’ (quats) have antimicrobial properties and are widely used as disinfectants. The antimicrobial activity depends on the quats’ ability to disrupt cell walls. They are not good against some gram-negative bacteria and of little value against fungal spores and non-enveloped viruses.
Quats can be very effective if used properly:
- Clean the area first.
- Use soft water, distilled or RO (reverse osmosis) water since hard water can make quats less microbicidal
- Apply directly to the surface, allow a short time for the quats to take effect, then wipe off.
Quats work as surfactants (more like a wetting agent). They do not corrode metal or damage plastics, unlike oxidative disinfectants and cleaners such as peroxide or bleach. They can potentially cause staining and leave a residue. However, some residue is desirable to maximise the antimicrobial effects. There are many commercial brands available which are cheap and effective hard surface cleaners for laboratory use.
When choosing a product, the following characteristics should be considered:
- A quaternary ammonium compound with the option of additives such as corrosion inhibitors
- Used for medical and general applications
- Does not stain or irritate the skin
- Has good materials compatibility with rubber, plastic, and metal without causing oxidation
- A bactericide, viricide and fungicide (Candida), effective within 5 minutes.
A good example of a combined cleaning agent and disinfectant for incubator shakers is Fermacidal D2 from IC Products SA.
Alternatives to Quats:
- Oxidative disinfectants such as bleaches: If these splash onto metal parts, especially bearings, they can cause corrosion. As a rule, do not use bleach in any device that contains metal components. It will damage everything from interior sheet metal to solenoid valves.
- Use of ultra-violet radiation to decontaminate the interior (some manufacturer provide as option for their incubation shakers).
- Hydrogen peroxide: This can permanently damage humidification sensors. As this can be used to decontaminate an entire laboratory or cleanroom areas, care should be taken to prevent access to the incubator chamber if a humidification option has been fitted. If possible, the humidification sensor should be removed or covered.
Cleaning of hard surfaces inside and outside the shaker
If a flask has broken or culture medium has escaped, clean the chamber immediately with a neutral household cleaner as a first step. Then clean with disinfectants e.g. quats, if necessary.
Depending on your laboratory guidelines cleaning of the outside surface should be part of a general laboratory routine (SOP).
Use a damp cloth and mild detergent to clean the touchscreen panel of the controller and the plexiglass cover protecting the lighting unit – do not apply liquid directly to the surfaces or use aggressive chemicals. Dry with a clean cloth.
There is a risk of damage to the surfaces due to use of abrasive cleaners such as scouring pads and similar utensils. Only clean the glass door inside and outside with a soft cleaning cloth and mild detergent solution (diluted with de-ionised water). Make sure no residues are left behind by rinsing with clean water.
Wipe the trays and their mounted parts regularly (rails, clips, adhesive matting, etc.) with a soft cloth and a mild household cleaner. If necessary, disinfect with a standard disinfectant e.g. quats.

Cleaning under the tray
If the area under the shaking table has a hygienic design, it will have a smooth base and is deep enough to hold spills from large flasks i.e. several hundred millilitres. Ideally, there is a drain, and this will lead to a nozzle for removing contaminated culture and cleaning fluids. After disconnecting main power, an operator should be able to reach inside to access all areas under the tray.
Depending on the shaker the parts of drive system inside the chamber range from simple counterweight to complex bearings and cog assemblies. If there is only a counterweight you can easily clean it by yourself, otherwise you will need help from a service technician for proper cleaning. Again, bleach should never be used as some of these parts can be uncoated metal in the interior of an incubated shaker.
If you encounter pieces of broken glass remove them carefully while wearing protective gloves. The culture fluid can then be wiped up with a cloth soaked in disinfectant. It is then safe to proceed to the next step.
A typical rinse:
- Carefully add hot water into the shaker ensuring it does not come in contact with the electrical system or fans, to avoid the risk of permanent damage
- Pour it carefully into the base of the chamber
- Use a large beaker to pour it into the incubation chamber
- Do not use a pressurised water hose for cleaning
- Drain it away through a drain hole, if present. If the drain outlet nozzle is not connected to the in-house wastewater system, push a hose onto the drain nozzle and let the water run into an appropriately sized container.
- Thoroughly dry the base area with paper towels
Cleaning of «Sticky Stuff» adhesive matting
Trays with adhesive matting need additional care. The adhesiveness of the mats will decline over time due to dust and soiling. They can be regenerated as follows:
- Scrub the surfaces vigorously with a scouring pad and clean warm water or mild soapy water (washing-up liquid).
- Allow to dry overnight. Do not use paper towels to dry the adhesive, they will stick!
- Disinfect with quats.
The structure and adhesive properties of the adhesive matting can be destroyed by solvent-based cleaners. This leads to a risk of flasks becoming detached from the adhesive matting during operation of the shaker. If the adhesiveness of the matting cannot be regenerated by cleaning, it must be replaced.

Cleaning the fans and electronics
Dust, fluff and loose items of detritus such as bits of aluminium foil from flask caps can all pose a problem. This applies to:
- Air vents, especially those for cooling the electronics
- Fans and associated heaters
- Cooling systems integrated with the incubator shaker
The cleaning method below should be applied:
- Disconnect from mains power.
- Carefully use a vacuum cleaner with fine nozzle attachment to remove any accumulated dust and debris. This should be a gentle process to prevent physical damage.
- External air vents can also be cleaned in the same way.
Cleaning the area around the incubator shaker
Dust and debris can accumulate around the shaker, flasks can be dropped in front of the shaker and liquid can spill under the unit. Good laboratory practice should deal with these situations, but a shaker in a corner of a crowded area may potentially become neglected.
Contract services may cover the cleaning of floors and work areas but will not include direct interference with laboratory equipment, especially if it’s in use. It is also very common for routine cleaning to be overlooked by research staff.
A best practise routine can be added to any SOPs for general cleaning and maintenance to risk manage these situations:
- Cleaning surrounding areas should be designated to a person, group or contractor
- A schedule should be agreed regarding frequency and thoroughness of the cleaning process
- A log should be kept detailing results of earlier untreated spillages
- If aggressive floor cleaners are used, it is made clear that splashes onto the equipment should be avoided or wiped off immediately

This overview is not intended to replace advice included in the operating manuals of specific incubator shakers from individual manufacturers, and company standards should supersede recommendations provided here. Basic methods and definitions have been provided which should be applicable in almost all cases. Any relevant comments, ideas for new posts and constructive criticism are always welcome.


Exotica leathers
Looking for the best way to clean your incubator shaker? Check out this fantastic guide from Infor's HT Discover top tips and expert advice to keep your lab equipment in perfect condition. Say goodbye to contamination worries and hello to optimal performance.
house washing companies near me near me
Your blog always provides a refreshing perspective. I appreciate the depth and effort you put into each post. Keep inspiring us!
maryia atalan
this article really help me . Thank U
Mary Albani
Please send pdf
Sarah P.
Hey,
could you send me this post as pdf document, so that I can print it and hand it out in the lab for the cleaning routine? That would be perfect. : )
Thank you very much,
Sarah
Valentina Haag
Hi Sarah
Thanks for your interest & amazing idea!
The e-mail with pdf is sent - you can generate your own pdf by printing the page with destination/printer "safe as pdf".
Don’t hesitate to contact your sales person for further support.
Yanick Parice
please send a quote for the following product: sticky pads for multitron. thanks
Chantal Fiechter Schaub
Hello Yanick,
the fastest way to get a quote is to submit a request on the product page itself. Our regional sales manager will then reach out to you to clarify the details.
Regards,
Chantal