Introduction
There are number of key elements to consider when choosing a device for biomass measurement. These include:
- Technology: OD measurement or backscattered light measurement
- Does your application imply high or low cell density?
- Probe choice
Two optical technologies are widely used to estimate biomass within a bioprocess: Spectrophotometry measured at a wavelength of 600 nm (commonly abbreviated as OD600) and backscattered light measurement. Backscattering light measurement allows for a more sophisticated monitoring process, the data is available immediately and changes in the culture can be observed in real-time. OD biomass measurement is performed offline, which may be easier to use when starting out. Deciding which one is the best for your process requires the knowledge of both working principles and how they influence the measurement.
Measuring Biomass Offline Using Optical Density (OD600)
Technology and Method
Measuring the optical density is a well-known, long time practiced offline technique, used and described in many publications. You need a spectrophotometer, which is commonly available in the laboratory. For a biomass measurement a culture sample is taken from the cultivation vessel and deposited into a cuvette. The cuvette is placed in the spectrophotometer inside a light beam with a fixed wavelength (usually 600 nm). The device measures the relative light intensity before and after the sample. Using the Lambert Beer equation, the difference between these values can be used to calculate the optical density of the culture.
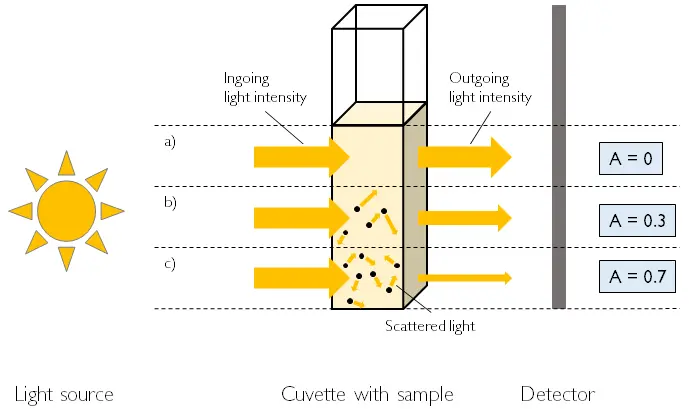
Measurement principle of the optical density. Options: a) no biomass, b) low biomass concentration, c) high biomass concentration. Increasing biomass also increases light scatter and decreases the intensity of light leaving the cuvette. Image: aquila biolabs 2020
Advantages and Disadvantages
Benefits:
- OD can be used for low cell densities and to monitor changes in low biomass concentrations
- You can measure the OD directly
- It is a precise, external measurement in specified spectrophotometers and cuvettes.
- It can be measured with equipment commonly found in the laboratory, no additional acquisition costs
However, the drawbacks are:
- Once the OD exceeds a value of 0.8 the sample must be diluted which increases the risk of errors due to pipetting and/or dilution
- Only individual cells can be measured, not filamentous organisms
- A sample will need to be taken from the culture. Therefore, the cultivation must be interrupted for every data point created.
- Interrupting the process influences the growth of the organisms due to lower oxygen intake and changes in temperature. Therefore, measured growth curves do not necessarily reflect growth of an uninterrupted cultivation.
- The number of cultures being measured will prolong the interruption time of the cultivation. Processing multiple flasks is laborious, and makes it difficult to minimize the time commitment or overall impact of stabilizing the culture process.
- An external device is still required
- The data will need to be transferred from the external device to a digital database
- No continuous measurement possible. As single samples are investigated, the data density is low
- The culture sampling process requires the shake flasks to be opened, which increases the risk of contamination
- The costs per experiment are low, but require additional man hours and therefore, increased labur costs. The workload is high and often done by overqualified personnel.
Measuring Biomass Online Using Backscattering Light
Technology and Method
A device comprising of a light source and a light detector comparable to the optical density is required for the backscattered light method. In contrast to the OD, the light source and detector are located on the same side of the sample. Light is emitted from the source and into the culture liquid. The photons interact with the cells and are scattered in different directions. Some photons return to the photodiode detector, converting the scattered light intensity into an electric current. Higher cell densities increase the probability of an incident photon interacting with a cell. With increasing cell density, more light is scattered back to the photodiode.
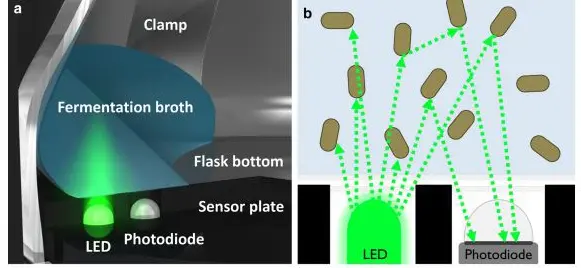
Backscatter light method. Source: aquila biolabs 2018
Advantages and Disadvantages
Benefits
- No sample has to be taken: no contamination risk and no interruption of the cultivation
- No sample dilution required, can be used to measure high cell densities
- Good method to estimate biomass concentration
- Biomass trends are immediately available
- Changes in biomass / growth can be seen directly, it can be responded to these changes within minutes
- Can be automated – continuously measurements possible and the possibility of human error diminished
- Continuous measurement can reveal features of culture metabolism that may not have been apparent with periodic sampling
- High data density
- Using an automated system, nights and weekends are undisturbed, as no researcher has to be available to create data
- Workload is very low
- Can be used for filamentous organisms
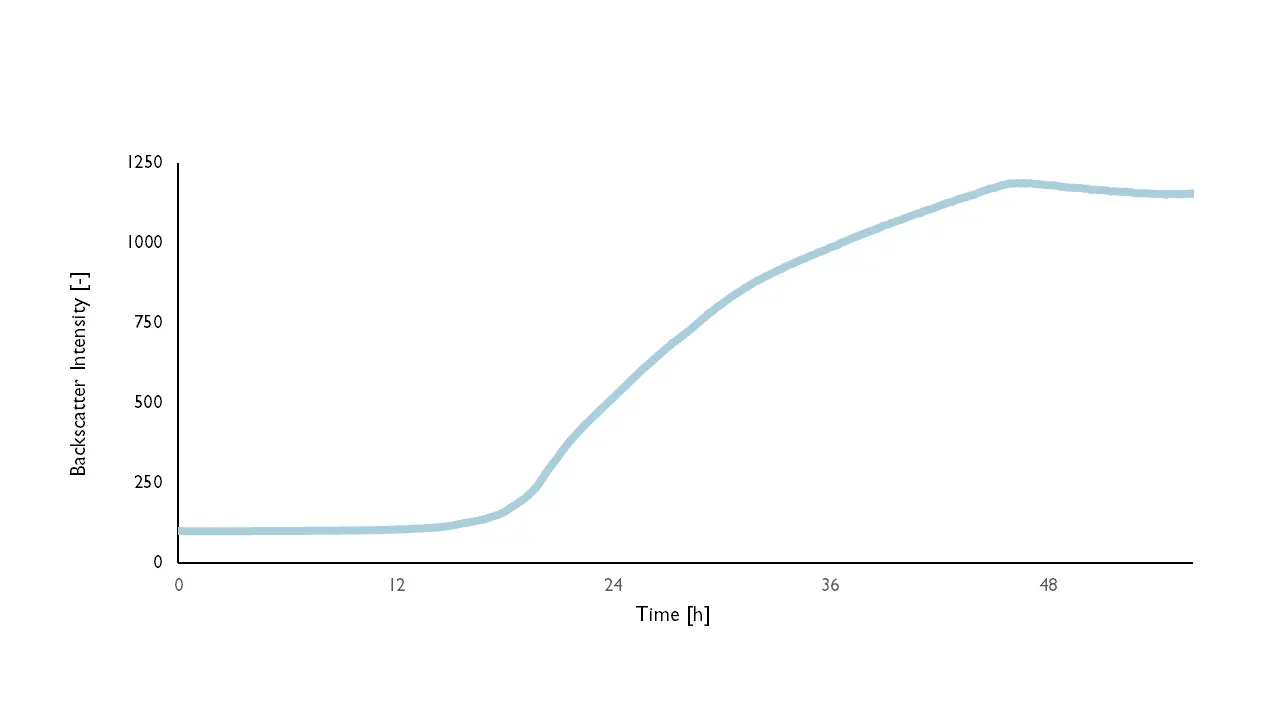
Continuous measurement of the filamentous organism Aspergillus niger using backscattered light. Source: aquila biolabs 2019
The drawbacks are:
- Despite increasing level of awareness backscatter values are still poorly established as a parameter. To compare these values with a known one like OD, a calibration has to be performed
- Resolution limit is higher than in a photometer – only “high” biomass concentration (OD 0.5 to 70) can be measured (no cell culture)
- Absolute accuracy may be affected as there is no external device with specially designed cuvettes are used but only bulk ware flasks in a shaken environment. Flask-positioning may therefore influence the measurements
- The device requires installation and can only be operated in this position
Comparing the technologies for biomass measurement
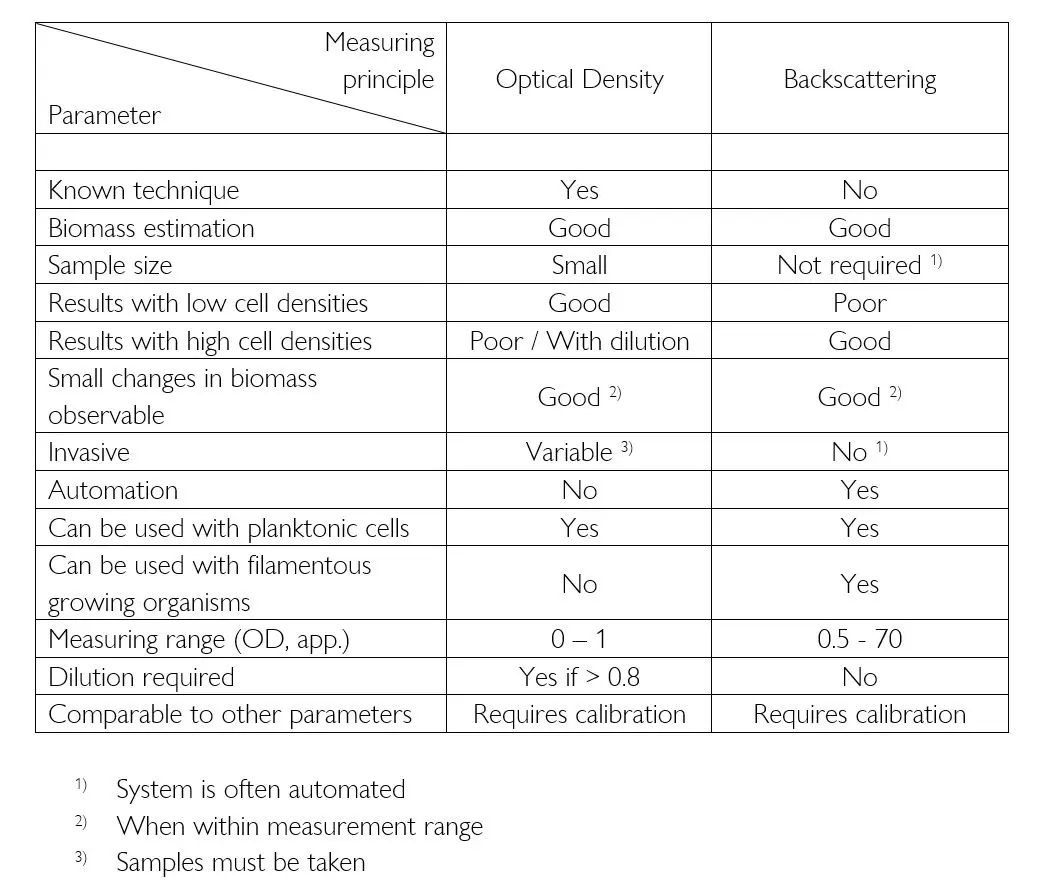
Summary
Both OD and backscatter are adequate methods of estimating the biomass in shake flasks. While OD is able to precisely measure lower cell densities and more common values, backscattering eliminates the need for dilutions and can be used with very high cell densities. Therefore, both technologies can be used equally as well when comparing external devices, depending on the focus of the cultivation.
The biggest differences can be seen when comparing the methods for taking measurements. Using an automated online system the data is available immediately and changes in the culture can be observed in real-time. This decreases the response time and prevents the researcher from sampling when nothing “interesting” happens. Online biomass measurement also provides a complete characterization of the culture, and there are no down-times on weekends or evenings, as there would be with offline measurements.
As seen in many other industrial fields, process automation is often desired as it saves time and eliminates human error. This will also mean purchasing additional equipment, which is where weighing up the pros and cons will influence your decision.
Bernd Leuchtle studied biology with a focus on biotechnology and biochemical engineering in Aachen, Germany. After acquiring his doctorate and a period as post doc, he joined the team of aquila biolabs as an application specialist.