The culture of the methylotrophic yeast Pichia pastoris is a standard microbe in recombinant protein bioprocesses. Commercial use in the 1970’s was aimed at using Pichia as an animal feed. As an expression system, it has several advantages:
- It can grow on a simple alcohol (methanol) as its sole carbon source.
- It can be used as part of an expression system typically switching on the Aox1 promotor for protein production after a period of growth on glycerol.
- It can grow to high cell densities, increasing the biomass per batch. Yield of protein can be in the gram per liter range.
- Protein is expressed into the medium, thus reducing the number of downstream processes is needed for its recovery.
- Due to its ability to partially glycosylate some proteins, it is being engineered to produce proteins normally found in animal cell culture
The cultivation of Pichia in a bioreactor comes with few challenges:
- It needs high rates of oxygen transfer to achieve high cell densities. This involves high speed mixing at high gas flow rates and/or supplementation with pure oxygen.
- The rate of growth and cell density is such that a lot of surplus heat is generated by the culture, in addition to that produced by the high-speed mixing. Heat output is greatest when a methanol spike occurs during feeding.
- As protein concentrations increase, foam formation may become a problem and dosing with an antifoam agent or use of a mechanical foam breaker may be necessary.
- Different feeding strategies may be needed.
The solutions to these problems depend on the capabilities of the bioreactors used. The following short overview will help to fill in some of these details.
High cell densities need a good oxygen transfer
High cell densities demand a high rate of oxygen transfer. For this a powerful motor sized to the vessel volume is needed, allowing high speeds to be reached even with large volumes. The size, type and number of impellors are also critical. Flat-bladed Rushton impellers of 0.33 the vessel diameter are optimal to work with baffles. Large bubbles from the gas sparger are broken into many smaller ones which increases the surface area for gas transfer. Durability and reliability of the drive system play a key part for Pichia fermentations as they have a typical duration of 60 hours. Over half of that time will be under conditions of maximum load.
The gassing capabilities are also important. Not just in terms of overall maximum volume but also the level of control. Integrated mass flow valves can supply both feedback and control for process optimization. The ability to supplement the air stream with pure oxygen can allow Pichia cultures to reach higher cell densities than it would be possible with air alone.
Key bioreactor components supporting oxygen transfer include:
- Flat-bladed Rushton impellers
- High-performance stirring system capable of up to 1200 min-1
- Integrated mass flow valves for gas flow control
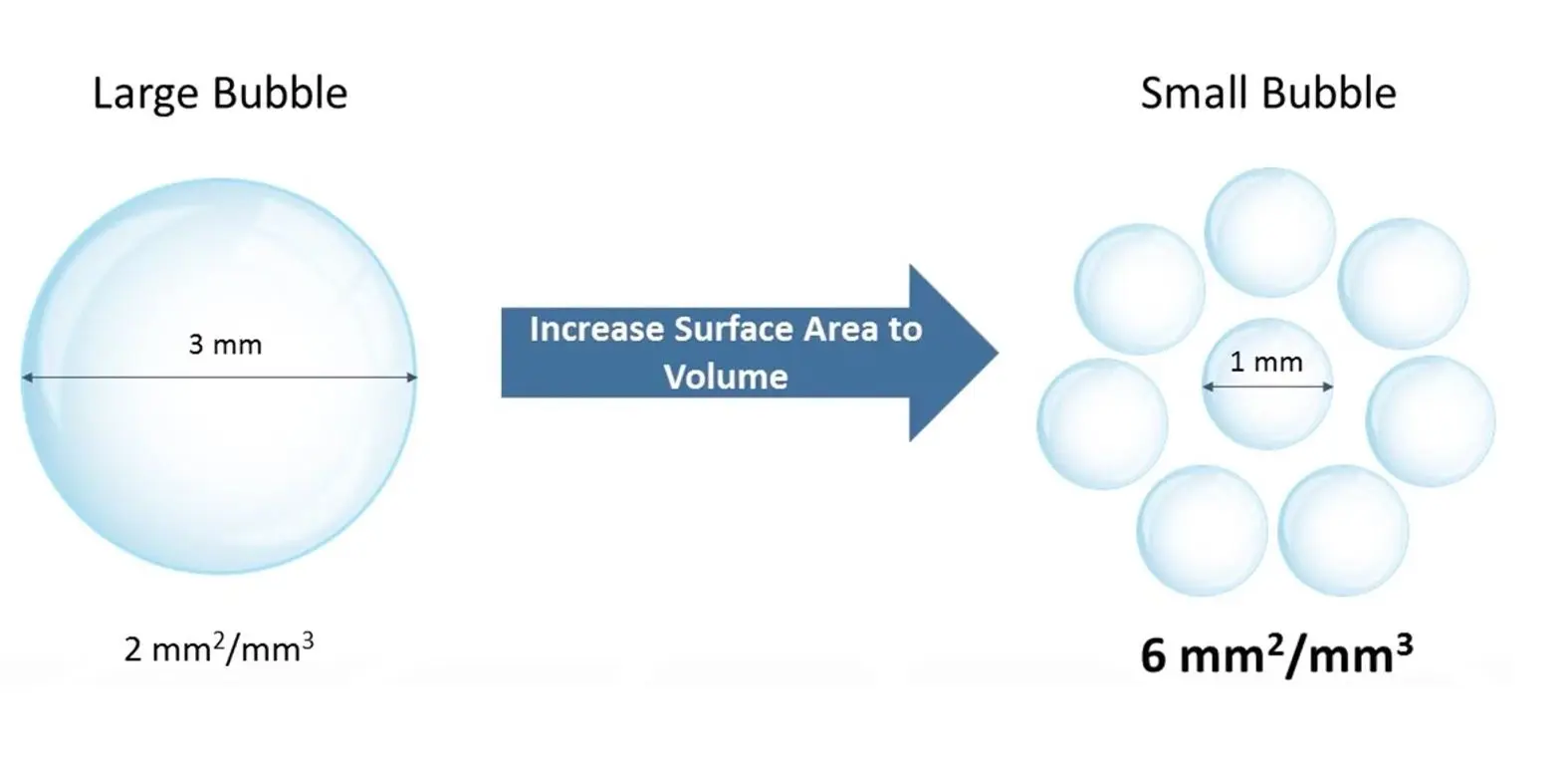
Comparing the gas-liquid interface of bubbles. Smaller bubbles have two advantages for oxygen transport compared with larger bubbles: A larger gas-liquid interface per unit of liquid volume as well as a longer residence time in the medium, allowing more oxygen to be transferred into the liquid.
Ensure excellent vessel cooling
The ability to cool the vessel against sudden, rapid increases in temperature is necessary for successful Pichia fermentation. This depends on the temperature difference between the culture and the coolant, the surface area in contact for heat transfer and the rate of flow through the cooling system. A recirculating chiller with a 50/50 propylene glycol coolant is almost certain to be needed for high cell densities and this could be run with as much as 20°C Delta T.
A jacketed vessel or an external thermal block can be used. If cooling is not adequate, a cold finger can be added to an available port to put the coolant in closer contact with the culture liquid. This could either be controlled by a modified temperature control loop to be turned on as needed or counterheating used if cold water flowed constantly through the cold finger.
Cooling options for pichia pastoris fermentation:
- Jacketed vessel or an external thermal block
- Recirculating chiller with a 50/50 propylene glycol coolant
- Cold finger
You need flexible feeding capabilities
The substrates used to feed the Pichia culture are the usual way of controlling the production of recombinant protein production. A growth phase on glycerol or glucose provides for the efficient production of biomass. The glucose/glycerol feed is then switched to Methanol, this switches on the AOX gene which triggers production of the recombinant protein.
Two pumps are needed to supply:
- Glycerol in the initial stages of the culture is needed to increase biomass without metabolic resources being diverted to product formation. To get a high culture density, this substrate needs to be delivered in both batch and fed-batch mode. This is usually followed by a period of starvation.
- Methanol is added as a substrate, which activates the Aox1 promotor to allow product formation to begin. The concentration of methanol may vary during fermentation, as the Pichia adapts to the new substrate. Too much methanol will be toxic.
A blending of the glycerol and methanol as an intermediate phase is also possible. Methanol may be provided in bursts, with starvation periods in between or feed rates kept close to starvation levels.
Therefore, peristaltic pumps are needed which are capable of exact delivery across a range of flow rates. A pump can be re-allocated to deliver a feed rather than e.g., antifoam. In this example, the loss of the antifoam pump could be mitigated by adding low levels of antifoam reagent into the feed, or by adding a mechanical foam breaker at the liquid/gas interface.
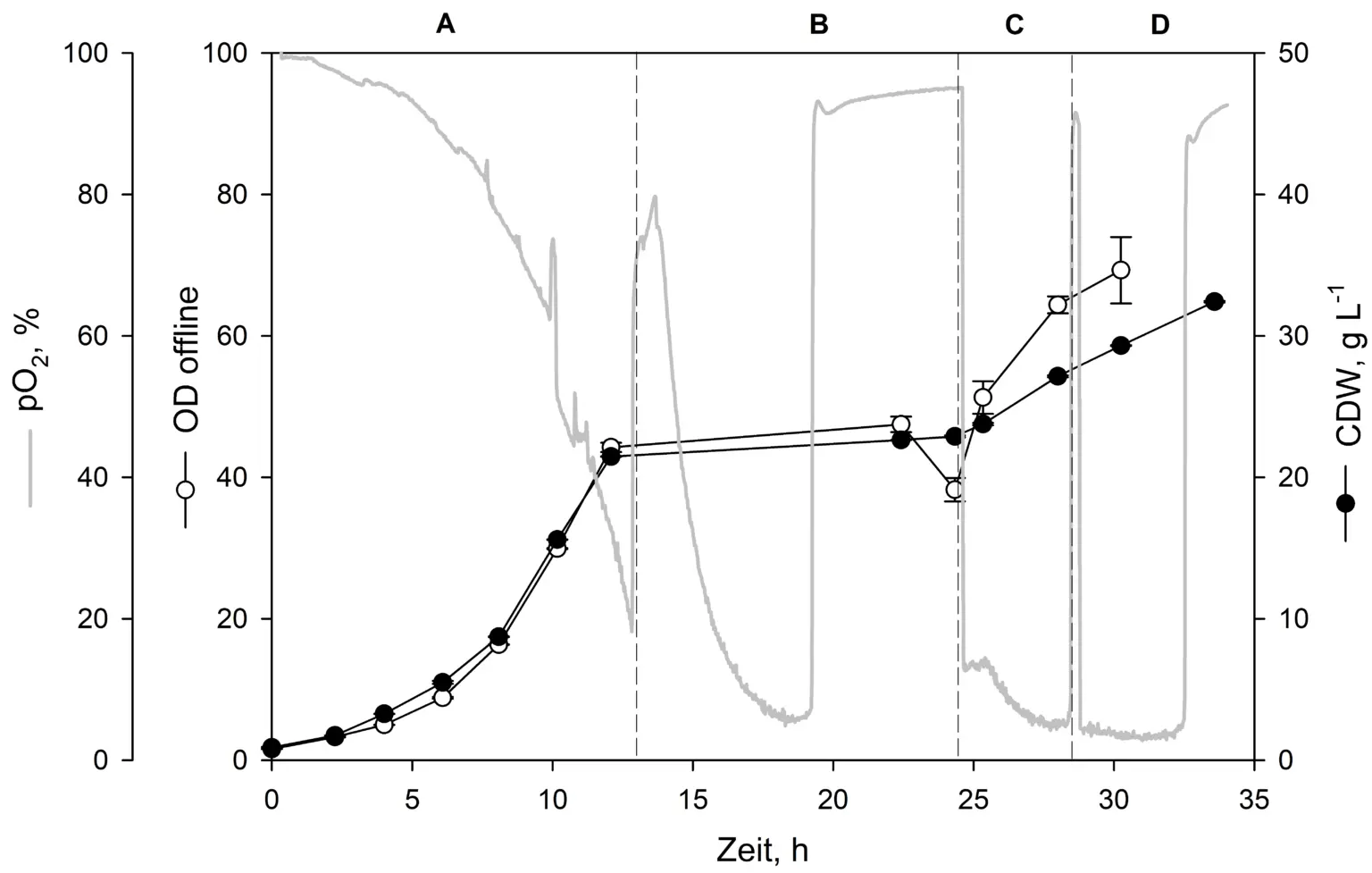
Process overview of a batch/pulse method showing biomass growth on glucose (phase A, 0 to 13 h) and subsequent induction via MeOH pulses (phases B-D, 1% v/v).
Use automated process control
The local control at the bioreactor mediated by the Human Machine Interface (HMI) will provide options for cascade control of dissolved oxygen across several different parameters. Control can begin by stirrer speed and then transfer to air flow when it has reached a user-defined maximum. The next step would be to use gas mixing to supplement the air flow with oxygen. This needs no user intervention and results in a carefully graded response to changing conditions.
Example cascade for control of dissolved oxygen:
- Increase stirrer speed
- Increase air flow
- Supplement oxygen to the gas mix
Furthermore, the use of bioprocess control software can take the degree of automation to a new level. It now becomes possible to look for spikes in dissolved oxygen (which shows all the substrate has been used by the cells) and use this to automatically change feeds and/or start pulsed feeding. Workflow control can divide the process into phases and have different operating conditions for each phase. These can be linear or looped to repeat certain steps.
In addition, physical sensors in or around the vessel can supply data on cell growth and metabolism. Soft sensors can use simple readings from the physical sensors to calculate other, indirect values more related to cell metabolism. These factors can then alter the control parameters in real–time.
Use sensors for a better measurement of cell densities
Extending the capabilities of a basic bioreactor with more sensors is a big advantage for Pichia culture. The two most used are:
- Optical density: The measurement is based on the absorbance of the culture at a specific wavelength (~600nm) and can be expressed in optical density or OD600 units. A probe is located inside the vessel to take the measurements in real-time. Optical density can show any problems with growth strategies or provide a trigger for starting the starvation phase when a certain cell density has been reached.
- Exit gas analysis: The analyzer takes a sample of gas leaving the bioreactor and measures the amount of oxygen and carbon dioxide present as percentages of the total gas mix. Since the amounts of these gases going into the vessel are known, the differences relate to cell metabolism. Sending these values to a soft sensor created in bioprocess software will allow metabolic parameters such as Respiratory Quotient (RQ) to be calculated. This can determine the efficiency of the metabolic processes going on inside the cells. As a final step, these calculated values can be used to control substrate feed rates and make automated adjustments over time.
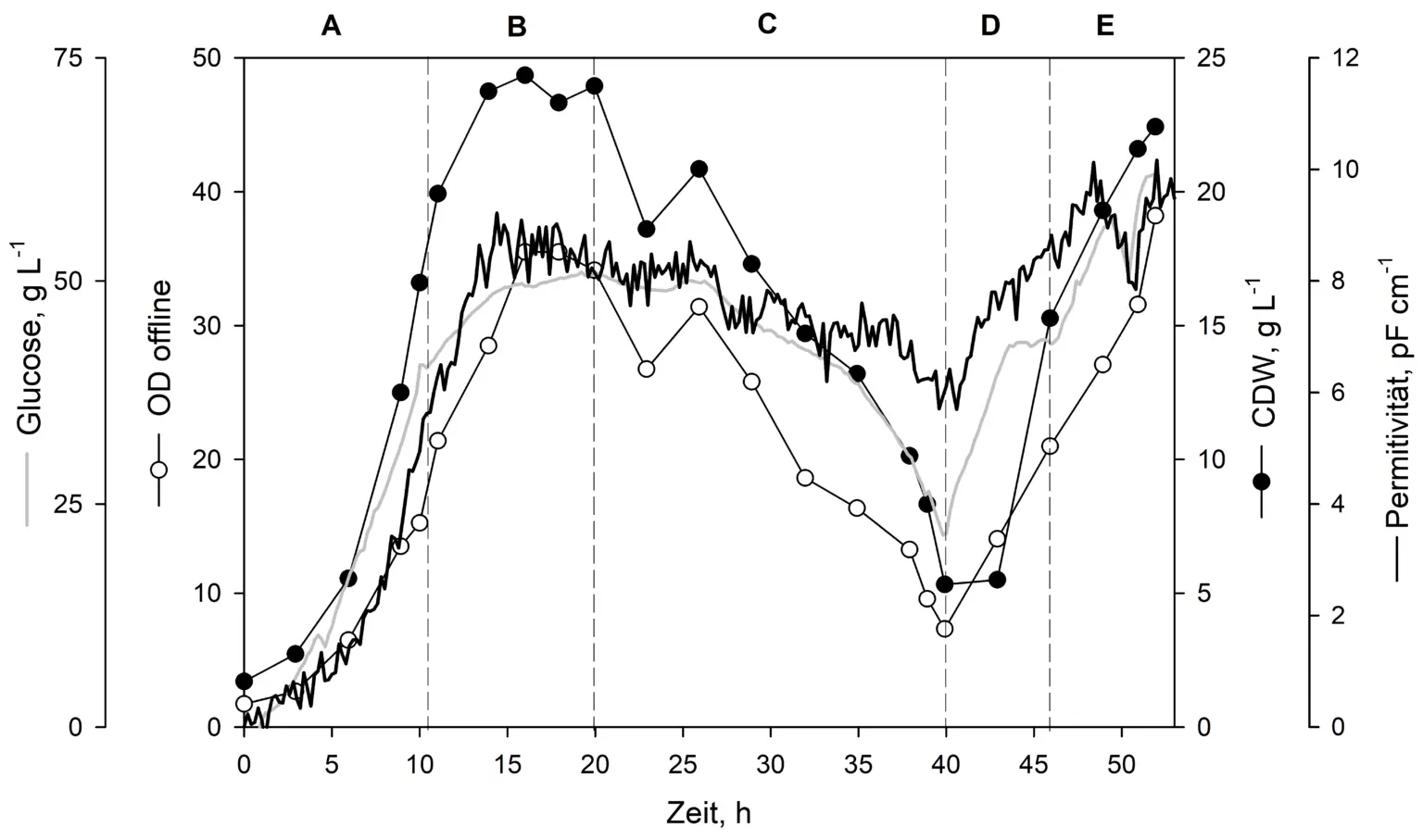
Comparison between online biomass-monitoring sensors in the Minifors 2 bioreactor during continuous cultivation. An initial batch phase (A) was followed by a phase with a constant dilution rate (B), a phase with a controlled elution step (C), a biomass re-enrichment phase (D), and a phase focused on product formation (E).
Conclusion: Several key factors are essential for successful fermentation of pichia pastoris
This has been a brief and simple overview of Pichia fermentation for newcomers. The main points have focused on what is needed for process optimization in terms of the capability of the hardware used.
The key factors identified as being of importance are:
- The need for a powerful drive motor and optimized impellor system to ensure high rates of oxygen transfer. Speeds over 1,200 rpm should be possible.
- The importance of efficient cooling for removing heat from high-density Pichia cultures
- A need for high accuracy pumps which can be re-allocated for Pichia fermentation “out of the box”
- The importance of both local and supervisory control software for automation and optimisation
- The value of additional sensors to detect cell growth rate and/or metabolic processes, especially when combined with soft sensors.