Low biomass levels can be attributed to:
- Oxygen limitation
- Lack of nutrients
- Cultivation interruptions and repeated handling
This post explains the various ways to increase the quality of biomass produced in your shake flasks by mitigating the causes of low growth and productivity. Individual experiments may only use some of these solutions, however, there is enough variety mentioned in this article to assist with a broad range of applications.
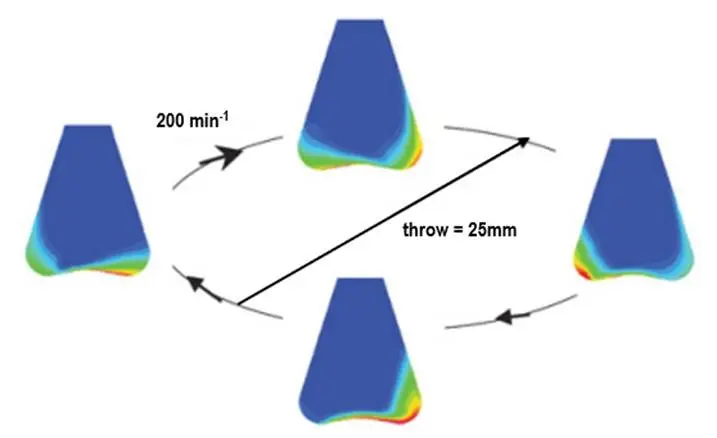
1. Choose the right shaking throw & speed for your flask
Often the reason for poor biomass concentration is not the clone, as you may think, but an external factor.
Two factors that have a big influence on the oxygen transfer and, therefore, the biomass growth is the shaking throw in combination with the shaking speed.
As a general starting point, 25 mm is always a good choice and can yield excellent results independent of the application. However, in some cases, restrictions in respect of oxygen transfer/cell growth will occur and a better result can be obtained if the shaking throw is selected according to the application.
As a rule of thumb:
- 25 mm throw: recommended for flask size from 25 mL up to 2 L
- 50 mm throw: recommended for flask size from 2 L to 5 L
Microtiter- and deep-well plates:
- Use 3 mm throw with shaking speeds of min. 800, up to 1000 min-1
Example of suitable settings for a microbial application (Bacteria, Yeasts, Funghi)
- Mostly 25 mm shaking diameter
- Shaking speed: 120 – 400 min-1, mainly around 300 min-1